An Essay On Water ; Know The Science Behind It
AN ESSAY ON WATER
WATER –
Hello readers, I hope all of you will like this Science fact in my article”an essay on water”, which is going to be very interesting for you. Yes, of course, you have seen already the title of the article “an essay on water “, and you must be thinking, it is quite common, and nothing seems interesting in it.

But, here I am going to tell you the science behind the water, which will be scientifically based and after getting the knowledge about it, you will feel happy and proud to be yourself.
You will see the water from different perspectives from now. At least a scientific angle you will get to know.
I will try to explain it in an interesting way so that you can also make a puzzle or teach it to your kids or small children to make them understand it.
WHY THIS IS COMMON FOR US-
We all know, How water is essential for us?. We can’t live without water for many days. Water is essential for all living beings.
Apart from this, we use water for many purposes like cleaning, doing household chores for maintaining hygiene, for factories, industries, for cooking etc.
But have you ever known the scientific fact about it?
If you have some basic idea about elements*, compounds* then you will understand this topic easily. Otherwise, you can see it at the bottom of this page.
- Water has been discovered as a chemical compound in the 18th century by Cavendish. I hope you are well aware of the elements like Hydrogen(H), Oxygen(O), Carbon (C), etc.
- Water consists of Hydrogen and Oxygen in the ratio of 2:1 by volume and 1:8 by mass.
This means what you see, that the water is colourless, odourless and tasteless liquid, as a physical property, but chemically it is made up of two atoms Hydrogen and Oxygen.
3. When an electric current is passed through acidified or alkaline water. For every volume of Oxygen two-volume of Hydrogen evolve.
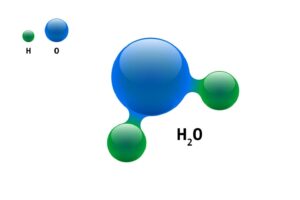
4. Water can be prepared by combining Oxygen and Hydrogen with the help of an electric current, where one part of Hydrogen and 8 part of Oxygen is required.
We boiled the water in our kitchen for making tea and for many purposes. We place the water in the freezer of our refrigerator.
But did we think? that why water remains as such in-room ?. Why does water need to be placed in the fridge for ice and needed to be placed on the flame for boiling?.
It is the game of temperature. In freezer and on the gas flame you will find the different temperatures, because-
5. The boiling point of water is “100” degrees Celcius and the freezing point are “0” degree Celcius.
If you put the water in these temperatures the water will boil and become ice at this temperature respectively.
DO YOU KNOW THERE ARE TWO TYPES OF WATER-
HARD WATER AND SOFT WATER
How did you find this Hard and Soft, as physically it looks the same?.
Hard and soft water depends on the mineral content dissolved in it.
SOFT WATER-
Soft water has less likely to have mineral contents.
HARD WATER-
Hard water has higher dissolved mineral content like Calcium and Magnesium.
All water, except Distilled Water, contain dissolved mineral.
PROBLEMS CAUSED BY HARD WATER-
If the water in your area is Hard water, which means Water has more mineral contents, it decreases its suitability for daily usage.
The question is, how do you recognise hard water?
Hard water is recognized from its action with soap. Soaps and detergent lather poorly in hard water. This means we can’t make enough foam with hard water, even after using more soap.
Moreover, hard water doesn’t clean properly. Many kitchen utensils or any household appliances are damaged through hard water due to their dissolved content of hard water.

It can clog the pipes and many things you can experience in your daily life while using hard water.
Hard water is also not suitable for drinking and irrigation purposes due to its higher mineral contents.
Read also…
EFFECT OF SYNTHETIC DETERGENT-
Some shower products and detergents that are meant for use in hard water have been specially designed. These soaps and shampoos remove hard water build-up and soften the water for bathing.
Apart from this, a water softener can also be used.
So that means if our area has hard water we are suffering through the bad consequence of hard water, so don’t worry about it.
There is a solution to it and we can save many utensils, appliances and many more. We can convert the hard water into soft water. This means we use a softener for this purpose.
The question is what is a water softener? how it works?
WATER SOFTENER-
A water softener is a device that uses sodium chloride( NaCl common salt) to treat hard water.
Minerals such as calcium, magnesium, manganese, and iron that are present in hard water are drawn up in the underground water supply and after heating, they crystallize and stick to utensil surfaces.

Sodium chloride, the affective component of water softeners, acts to remove these unwanted minerals.
The hardness of water is of two types-
-
TEMPORARY HARDNESS-
Which is due to the Bicarbonates of Calcium and Magnesium. They can be removed by
1) boiling
2)addition of lime.
This means, if we boil the hard water or if we add the lime in the hard water, we can surely remove the temporary hardness of the water.
-
PERMANENT HARDNESS-
This is due to the Sulphates and Chlorides of Calcium. They can be removed by
1)addition of washing soda
2)distillation.
If we add washing soda in the hard water and if we make the hard water undergo the process of distillation, then we can remove the permanent hardness of the water.
Read also…
RAINWATER-
Rainwater is the purest form of water since it is the condensed water vapour of the air. It is soft water because it does not contain salt like Bicarbonate, Sulphates and Chlorides of Calcium and Magnesium.
RIVER WATER-
River water by flowing over the Earth’s surface carries with it soluble minerals of the Earth and becomes hard water. It also contains several pollutants.
CONCLUSION-
Now I am sure you all will have the knowledge about water and liked my article”an essay on water” and you will be able to know the scientific angle of water.
And now you are seeing the water from a different perspective. Right?.If you are observing your utensils, tap, pipes at your home.
You are observing the amount of lather formation, then you are absolutely clear whether it is soft or hard water coming into your area. I hope you liked my article”an essay on water”.
ELEMENTS*-
A substance that can not be broken down into simpler components by any non-nuclear chemical reaction.
COMPOUNDS*-
Something that consists of two or more things or substances combined together.
ACIDIFY*-
To become acid or make something become an acid.
ALKALINE*-
A chemical substance that reacts with acids to form a salt. An alkali has a pH value of more than 7.
Read also…
